About Signatera™
The Signatera™ test is a highly sensitive and personalized molecular residual disease (MRD) test that uses circulating tumor DNA (ctDNA) for analysis. The Signatera™ test is custom designed for each patient to help identify relapse earlier than standard imaging procedures.

What is molecular residual disease (MRD)?
How can Signatera™ help?
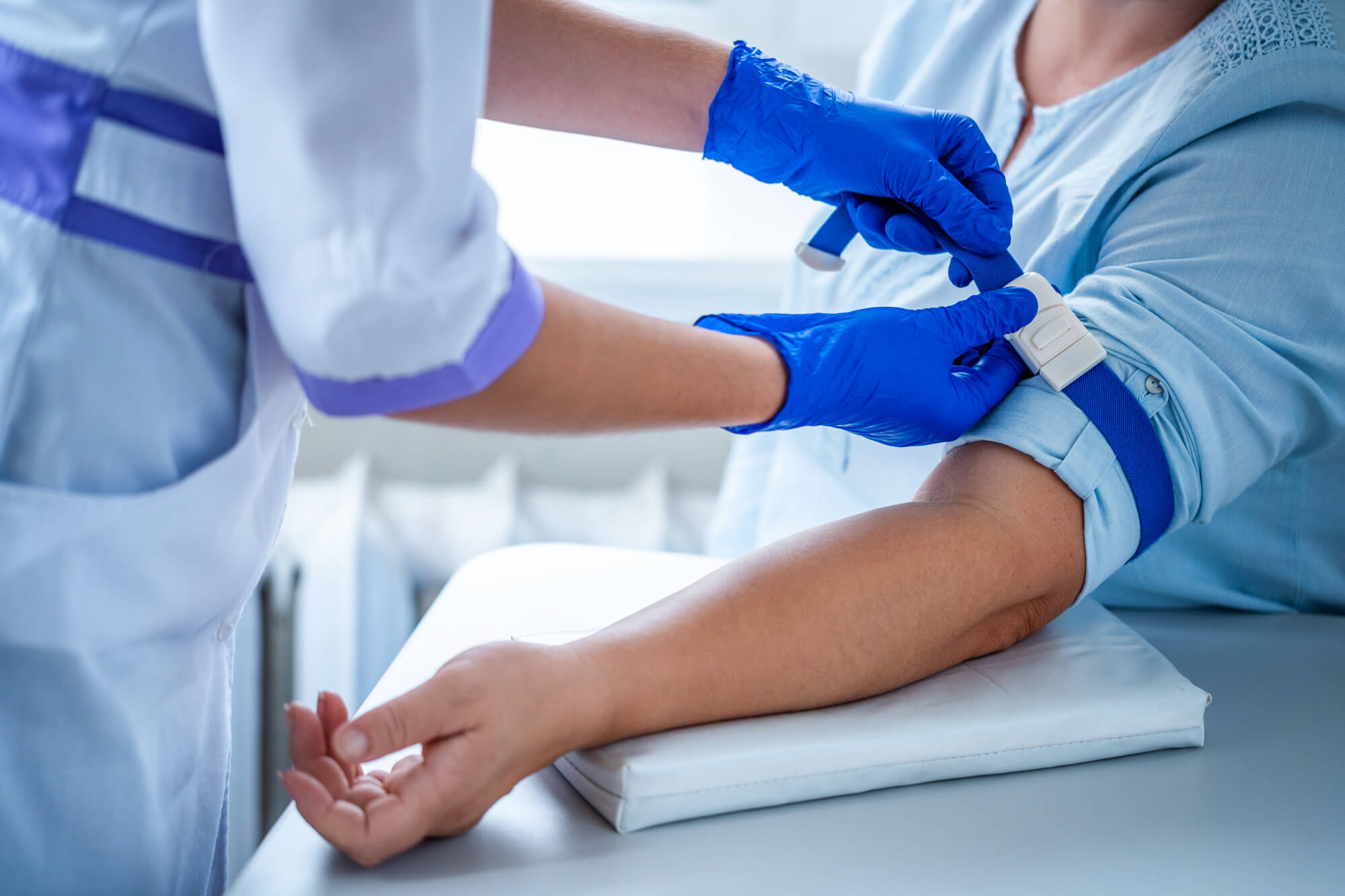
How the Signatera™ test Works
The Signatera™ test is a personalized, tumor-specific genetic test for the detection of molecular residual disease (MRD).
As a first step, Signatera™ analyzes both your blood sample and a sample of the original tumor tissue to determine your tumor’s unique mutation profile. These unique mutations help create a custom-designed test, in approximately 3-4 weeks.
Blood samples are then taken at a regular basis to assess changes in ctDNA levels over time.
Approximately one week after each blood draw, you will receive your Signatera™ report and can discuss the results with your oncologist, analyzing how they can affect your further treatment.
What is Signatera™ used for?
Signatera™ can help to answer important clinical questions such as:
- Is there are any signs of cancer remaining in the body after surgery?
- Is the treatment working?
- Has the cancer returned?
Signatera has been validated for a wide range of tumor types.
The Signatera™ test is can be used for all solid tumors.
- Signatera™ test is designed using the patient’s own tumor tissue, making it highly sensitive and able to detect even microscopic tumor fragments in the blood samples
- Early detection of changes in disease burden can help make more informed decisions about further potential anti-cancer treatments
- Signatera™ uses ctDNA dynamics (the changes in ctDNA levels) to assess disease burden in real time – this means it can detect tumor progression earlier than conventional imaging methods
Signatera™ can help plan treatment for cancer patients
Meaning of the Signatera™ results
Understanding the Signatera™ results
Positive Signatera™ test
Circulating tumor DNA of the monitored tumor can be detected in the blood sample
If the test result is positive, the chance of recurrence is high (>97%)
Negative Signatera™ test
Circulating tumor DNA of the monitored tumor cannot be detected in the blood sample
If the test result is negative, there is a high chance of lasting remission.
The results of continuous testing performed at regular intervals are represented in time or, if the result is negative, whether the level of ctDNA in your blood remains undetectable. With a negative ctDNA result, the chance of recurrence is low.
If the test result is positive, the chance of recurrence is very high – in this case, your doctor may recommend closer imaging monitoring or switch to a more sensitive imaging modality (e.g. PET/CT or MRI) from the modality used so far.
This allows earlier detection of relapse and increases the chances of successful anti-cancer treatment. Early detection is key to the successful treatment of malignant tumors. Early intervention prevents disease progression, tumor volume growth, and reduces tumor burden.

